In today's electronics manufacturing industry, many companies are using lead-free solder alloys. However, there are a few sectors in the industry that prefer the reliability and performance of tin-lead alloys. I've recently received a number of questions from customers asking how to adjust their solder pot to get the desired tin-lead percentages. Fortunately, Indium Corporation offers a large array of tin-lead alloys.
Here are three ways to change the composition of your solder pot:
- Dump the current solder pot and start with a fresh alloy that has the desired tin-lead constituentsd. However, this is not advised for the following reasons:
- Costs associated with downtime, labor, and materials
- Safety concerns with emptying a solder pot (the less times you have to clean the pot the less risk of injury)
- It is unnecessary
- The Additive Method uses pure tin or lead to increase the amount of tin or lead needed for soldering in the pot.
Before you start, here is what you need to know:
-
Weight of the solder currently in the pot
-
Percentage of tin and lead currently in the system
-
Percentage of tin and lead that you want in the system
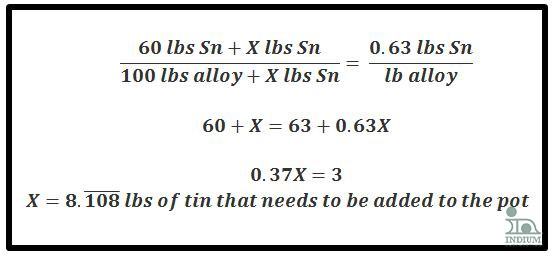
Ex 2a) Additive method (when the Sn% increases)
Given: 100 lbs of an alloy
Starting alloy: 60Sn/40Pb (60lbs Sn, 40lbs Pb)
Ending alloy: 63Sn/37Pb (63lbs Sn, 37lbs Pb)
Ex 2b) Additive Method (when Sn% decreases)
Given: 100 lbs of an alloy
Starting alloy: 63Sn/37Pb (63lbs Sn, 37lbs Pb)
Ending alloy: 60Sn/40Pb (60lbs Sn, 40lbs Pb)
3. The Subtractive Method requires that you remove some alloy from the pot and replace it with either 100%Sn or 100%Pb. When you remove some of the alloy, you also remove some of thematerial that you want so the calculation is different from the above two.
Here is what you need to know:
- Weight of the solder currently in the pot
- Percentage of tin and lead currently in the system
- Percentage of tin and lead that you want in the system
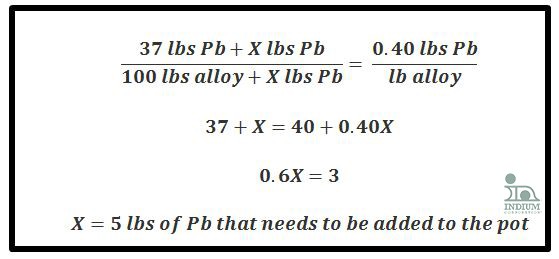
Ex 3a) Subtractive Method (When Sn% increases)
Given: 100 lbs of alloy
Starting alloy: 60Sn/40Pb (60lbs Sn, 40lbs Pb)
Ending alloy: 63Sn/37Pb (63lbs Sn, 37lbs Pb)
The first step is to figure out how much Pb needs to be removed by figuring out how much Pb you want in your pot and subtracting that from the amount of Pb that is currently in the pot:
The second step is to figure out how much of the alloy contains 3lbs of Pb:
This shows that in 7.5lbs of alloy there are 3lbs of lead. Next, you need to remove 7.5lbs of alloy and replace it with 7.5lbs of pure tin to get the desired 63Sn/37Pb alloy.
Ex 3b) Subtractive Method (When Sn% decreases)
Given: 100 lbs of alloy
Starting alloy: 63Sn/37Pb (63lbs Sn, 37lbs Pb)
Ending alloy: 60Sn/40Pb (60lbs Sn, 40lbs Pb)
The first step is to figure out how much Sn should be removed by figuring out how much Sn you want in your pot and subtracting that from the amount of Sn that is currently in the pot:
The second step is to figure out how much of the alloy contains 3lbs of Pb:
This shows that in 4.76lbs of alloy there are 3lbs of tin. Next, you need to remove 4.76lbs of alloy and replace it with 4.76lbs of pure lead to get the desired 60Sn/40Pb alloy.
Unfortunately, the Additive and Subtractive Methods can be inconvenient if you have to calculate percentages other than the ones above. This is why I created a calculator in Microsoft Excel that does all the work for you. All you need to do is input the current percentage of tin, the desired percentage of tin, and the weight of the alloy in the pot and it will calculate how much tin or lead you need to add to the system to achieve the final alloy. Please feel free to email me and I will be happy to send it along.
Have a good one,
Adam