Alloys that contain gallium have the intriguing property of remaining in the liquid state at room temperature and below. While pure gallium melts at 30°C, alloying gallium with indium, tin, and/or zinc lowers the melting point even further.
The chart below shows the melting point behavior of alloys that we offer:
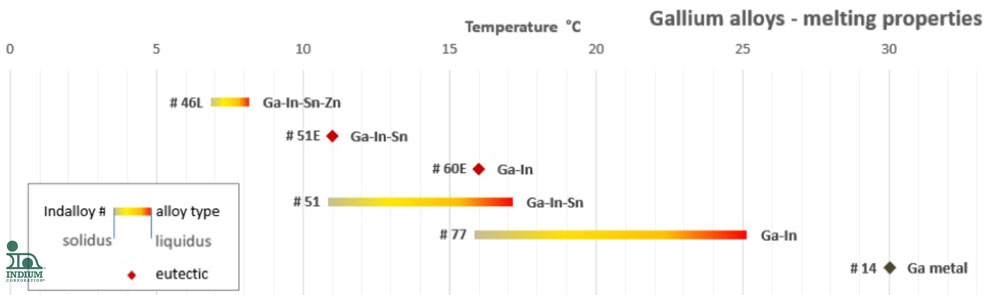
Indalloy® 51E is a eutectic composition of 66.5Ga/20.5In/13Sn with a melting point of 11°C.