Folks,
I was surprised to see the wrong formula for metal alloy density calculations on YouTube. The wrong (Eq. 1) and correct (Eq. 2) formulas are shown in Figure 1. In many cases, Equation 1 will give an answer only a few percent off. However, in some cases it can be off by more than a factor of 1,000 as seen in a past blog post. This blog post also gives the derivation of Equation 2.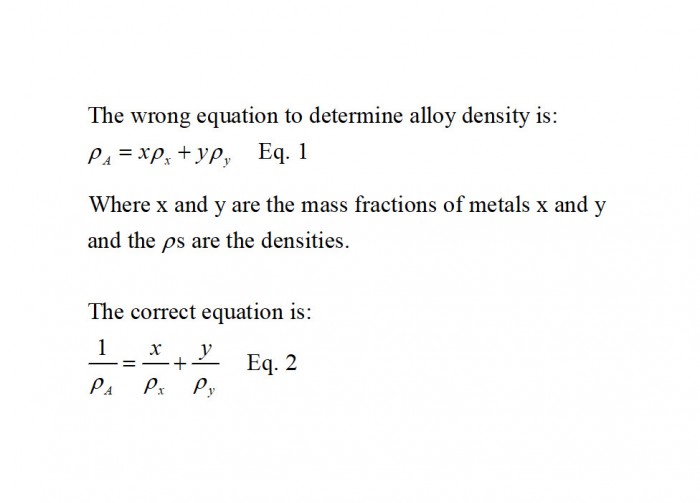
Figure 1. The wrong and right equations to calculate alloy density.
The YouTube video mentioned above did suggest one interesting task—determining the metal mass fractions of a two-metal alloy, while only knowing the alloy density. Of course, we know the densities of the two metals. The solution to this problem is seen in Figure 2.

Figure 2. Solving for the mass factions of two metals in a two-metal alloy.
To check the result, assume we have a tin-lead metal alloy. The alloy density is 8.4 g/cc. Tin has a density of 7.29 g/cc and lead 11.34 g/cc. By plugging these numbers into the solution for x (tin), we get 63% and y = 37%. Hence, this alloy is the tin-eutectic alloy.
This technique can solve only two-metal alloy system mass fractions.
Cheers,
Dr. Ron