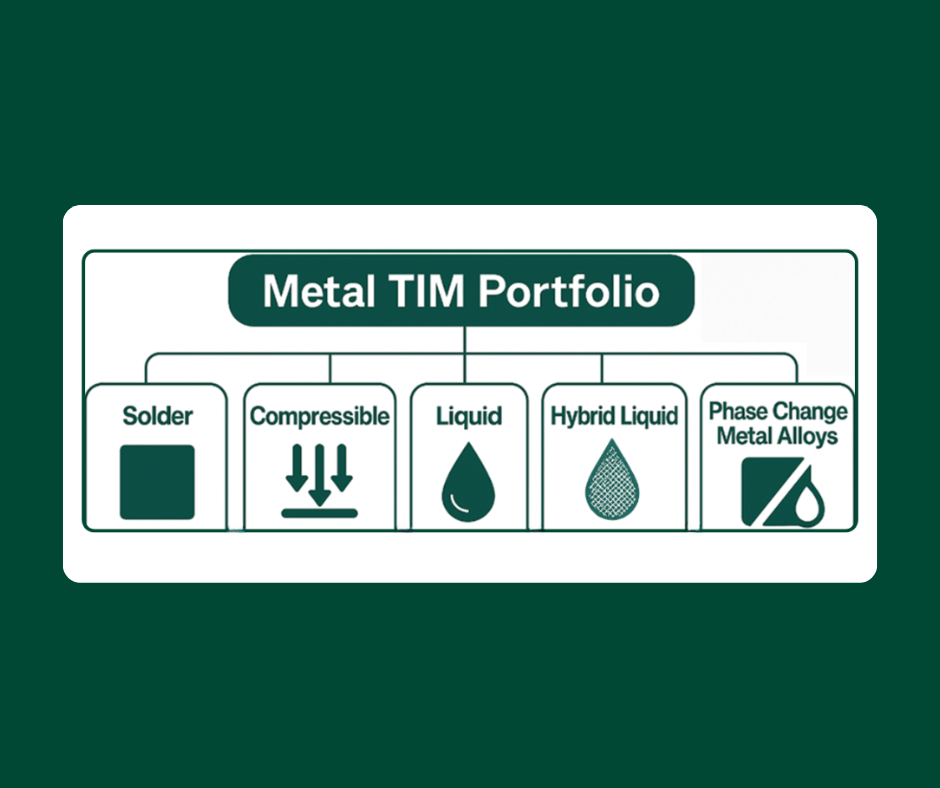
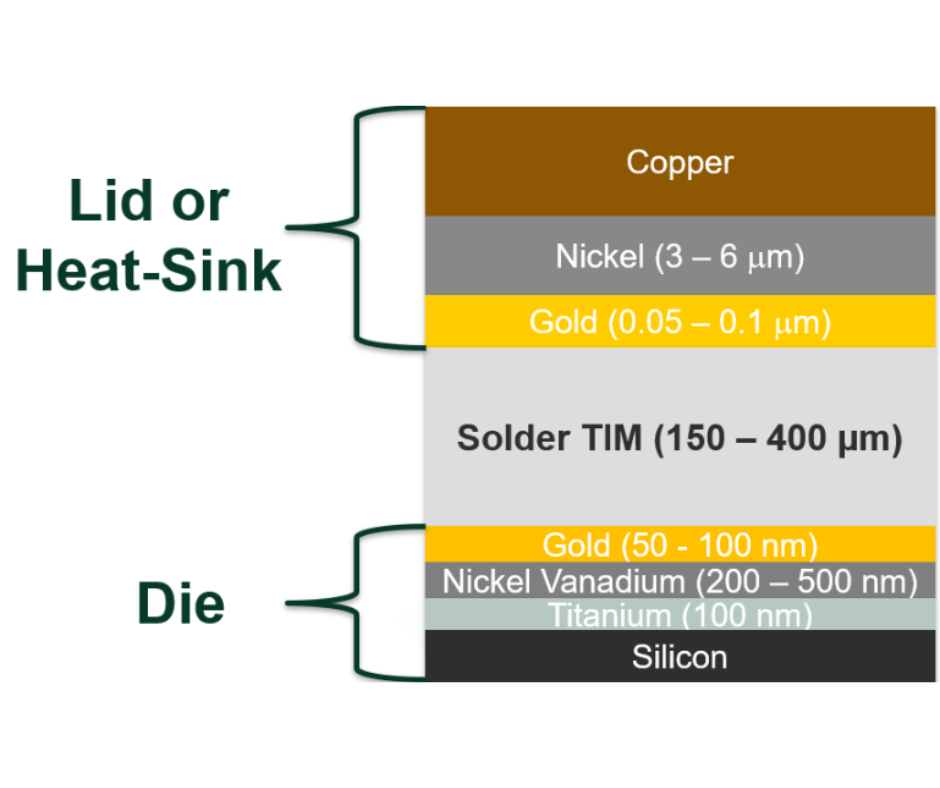
Bob Jarrett gave an excellent explanation of the thermal conductivity of metal alloys and their relationship with electrical conductivity. It is as follows:
The high thermal conductivity of metals is a result of energy transfer by free (or valence) electrons. These mobile electrons conduct electricity and thermal energy. For metals, the ratio of the thermal conductivity to the electrical conductivity is the Lorenz constant or the Weidemann-Franz ratio (L = κ/σT ~ 2.45E-8 WΩ/K²). When metals are mixed to form an alloy, the energy states of the free electrons are modified as the different elements have differing affinities for the wandering electrons.
The electrical and thermal conductivity of the mixture often deviates (negatively) from a linear rule of mixing. For example, Sn-Cu forms two intermetallic compounds (Cu3Sn and Cu6Sn5). The conductivity relationship still holds, but both the thermal and electrical conductivity are less than either constituent. Similarly in the Sn-Ag system, the conductivity of the 96.5Sn-3.5Ag eutectic composition is lower than either element.