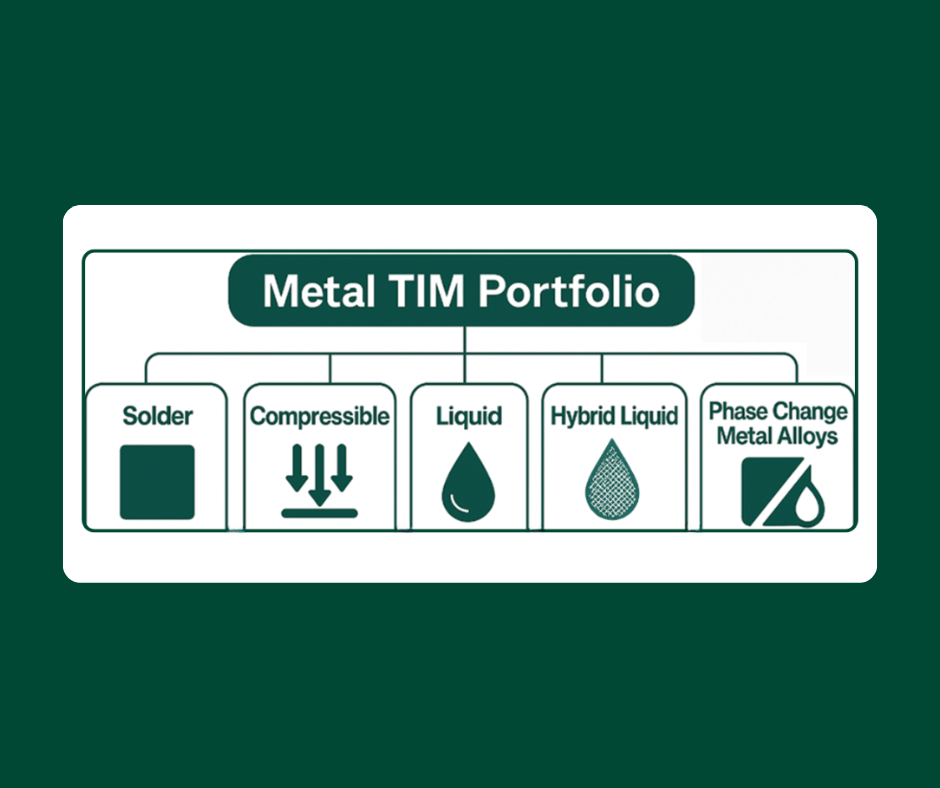
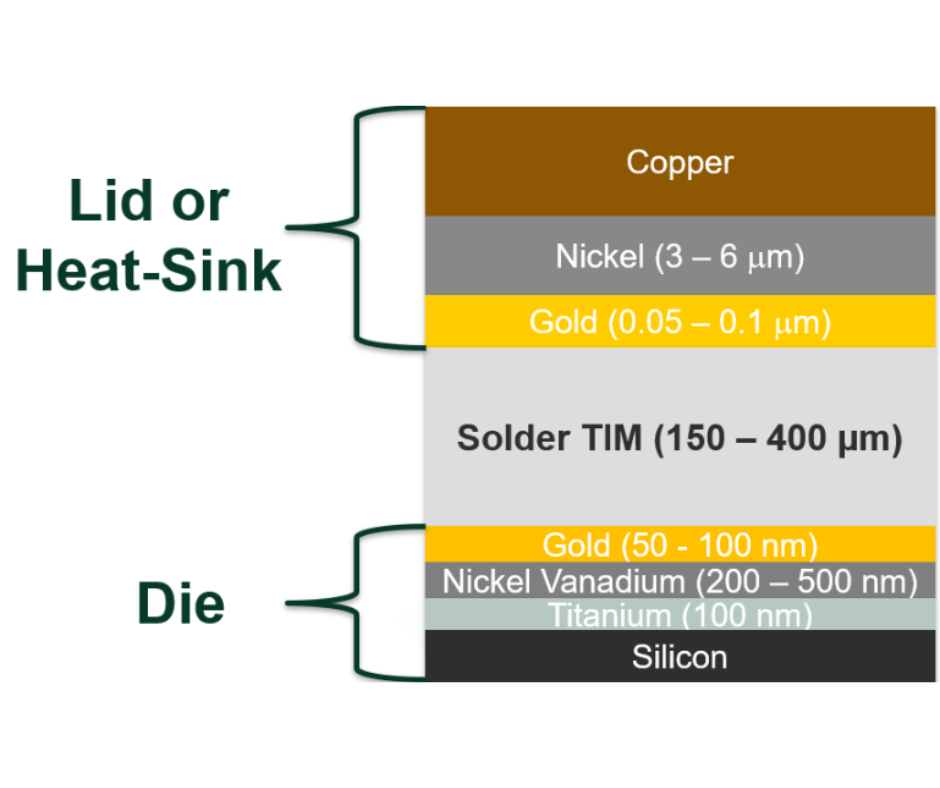
In our first review of our Heat-Spring® metal thermal interface material product line, we noted that the Heat-Spring® sprang from customer need. Polymers and greases were not good enough for the for high performance thermal management requirements of many of our customers. Indium metal has a high thermal conductivity (86W/m-k) but silver, gold, and even aluminum, have higher thermal conductivity. So, I went to Bob Jarrett, one of the designers of the Heat-Spring, to find out why indium works better than these other materials.
He told me there are two key characteristics that give indium the advantage over these other metals: Temperature and Flow Stress.
Indium melts at a temperature of 157°C. The other three melt at close to, or over, 1,000°C. "Indium meltsat 157°C, which is low enough to allow die-attach of the lid to the die without heating above reflow temperatures of the SAC solder used on the package. This low melting point also means that indium is annealed at room temperature – it returns to the softened state without heat," according to Bob. So, you can use an indium thermal interface as an attach material without destroying temperature-sensitive components or disturbing other solder joints on your board.
Flow stress simply means that indium is soft enough to do two things that other metals cannot:
- Compensate for a mismatch of the thermal expansion between two mating surfaces. Different materials expand and contract at different rates, creating a need for some compensation in any intermediate layer (like a thermal interface material). The other candidates are not as forgiving as indium. "Indium is really, really soft. Its flow stress is isabout 150psi (1MPa). This allows indium to conform to the mating surfaces and to absorb deformation due to differential thermal expansion without transferring the shear forces to the delicate semiconductor materials," says Bob.
- It will also optimize thermal conductivity by its increased contact even on imperfect surfaces. Bob adds, "Indium conforms to an asperity, bump, or dip in the interface surface where a harder material would simply lose contact around such obstacles. Indium maximizes the contact surface area."
Bob continued, "Indium's softness also provides the Heat-Spring® withthe inherent property that its thermal resistance decreases with time. The peaks in the Heat-Spring's patterns initially deform plastically by the load applied. Over time, the indium conforms to finer and finer asperities, increasing the contact area. A grease thins out over the entire surface (and pumps out the excess to the edges). The Heat-Spring's peaks flatten and partially fill the valleys, but they remain in the original location."
Next time we will explore thermal resistance and thermal conductivity.