Folks,
Suresh writes:
Dear Dr. Ron:
Can densities of less than 1 g/cc be measured with the “Wet Gold” technique?
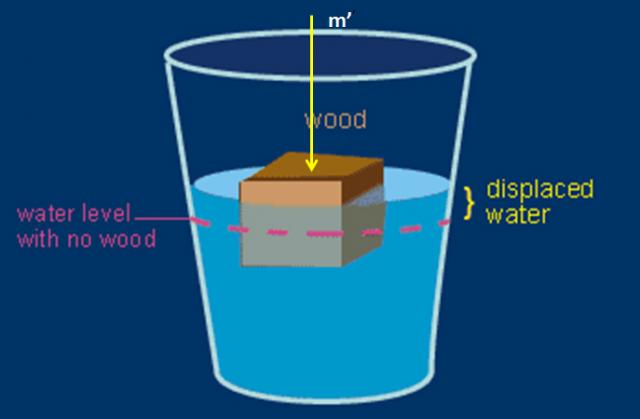
Well, to tell the truth, I didn’t know. But, after a little thinking, I developed a technique that will work. Figure 1 shows a wood block floating in water in a beaker. The volume of the block that is submerged we will call vs and the volume of the block that is above the water will be va. The total volume of the block is v. Assume that we have weighed the block of wood before putting it in the water and its mass is m. We then place the beaker on a scale and zero the scale. Next, we carefully use something like a pencil to push on the wood until it is just totally submerged. The yellow arrow in Figure 1 would be where the pencil should be pushing. The scale will now read an equivalent mass that we will call m’.
Figure 1. The setup to measure the density of wood. The yellow arrow is the point where a pencil should push on the wood to submerge it.
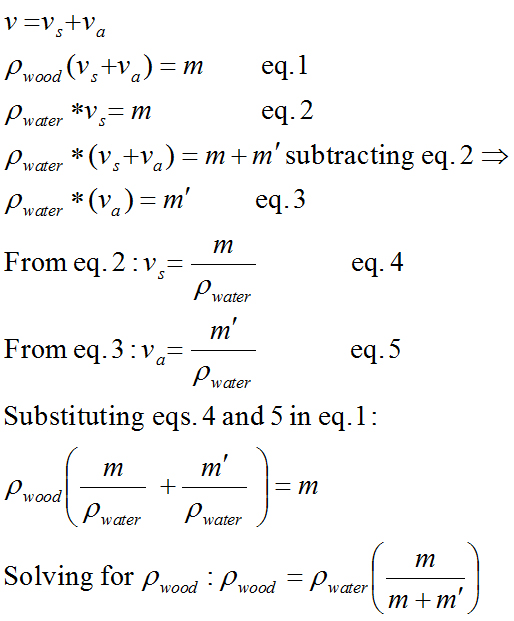
The density of the wood is then: densitywood = densitywater (m/(m+m’)). The derivation is shown below in Figure 2. In this derivation density is given by the Greek letter rho.
Figure 2. The derivation of the equation for measuring densities of less than 1.
I demonstrate the “Wet Gold” technique in my Dartmouth class, “Materials: The Substance of Civilization” . It works, but it is hard to get very precise answers. I would guesstimate the accuracy at +/-5%.
Cheers,
Dr. Ron