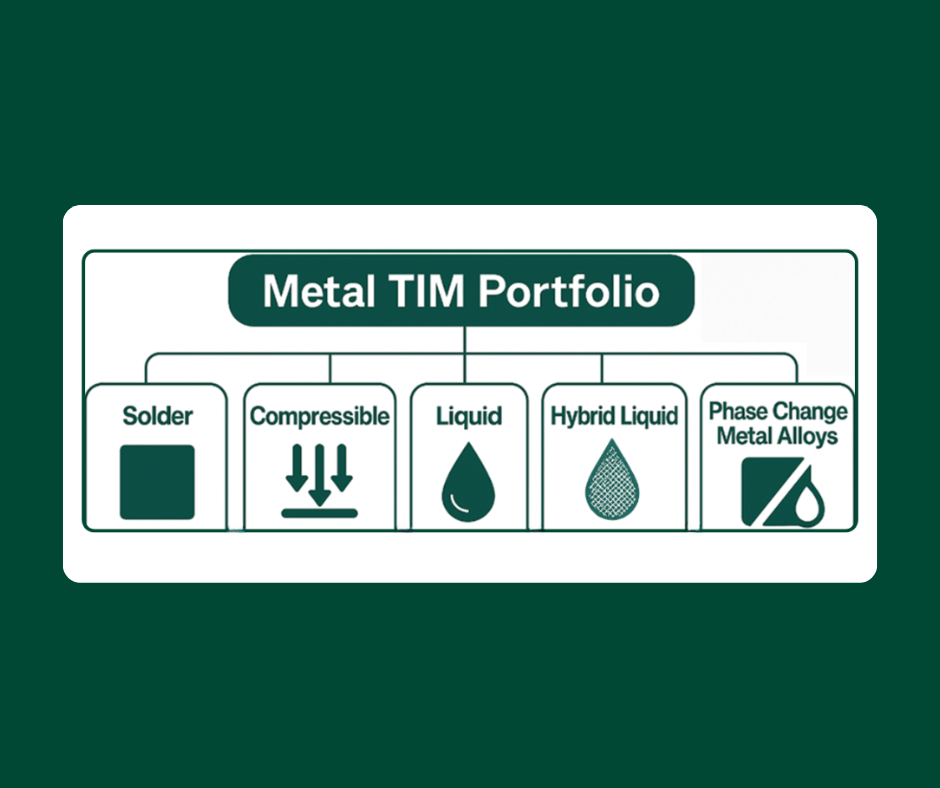
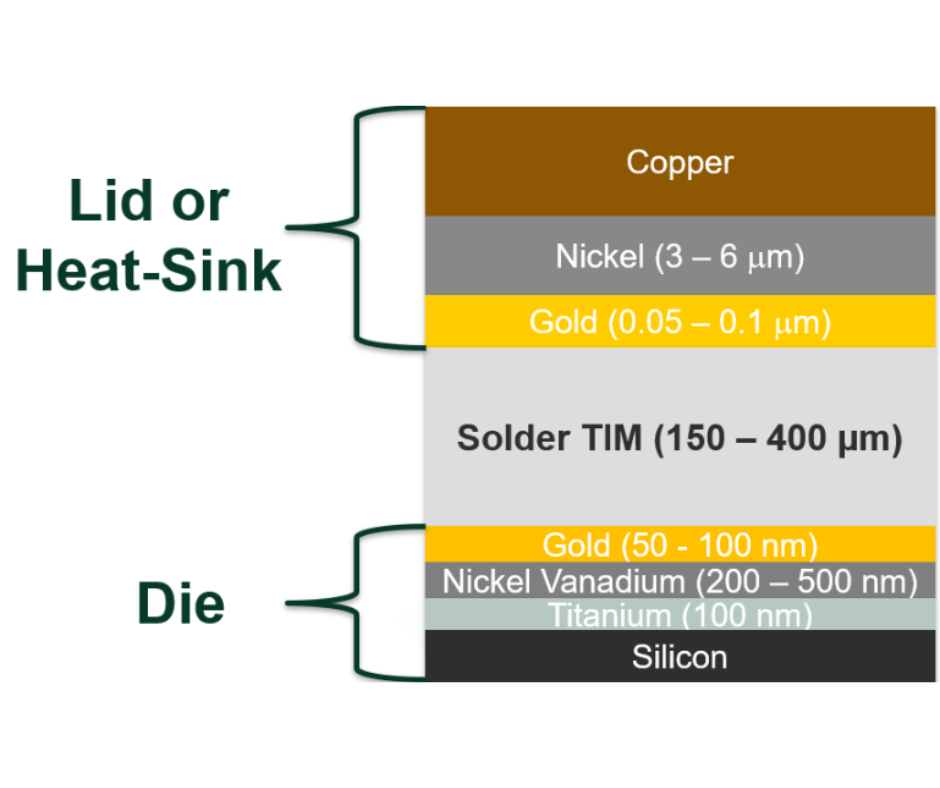
Soldering without flux is generally regarded as difficult if not impossible. Yet a fluxless process is routinely done with 80Au/Sn ribbon and preforms – how can this be?
The short answer: because it can be processed as a braze.
Let’s remind ourselves of the role of flux. Flux is a chemical which cleans (deoxidises) surfaces and protects it from re-oxidation during soldering so that the solder will wet and form reliable solder joints. Gold doesn’t tarnish. So, in theory, we don’t need flux. However, in practice, we rarely solder to pure gold versus gold-plated surfaces. Unless it is very thick, the Au plating (for example: flash Au < 0.5 microns thick) rapidly dissolves into the AuSn, and the solder joint is actually made with the base metal which lies beneath the Au. This is almost always nickel. Unfortunately, nickel does oxidize and can do so even when plated with gold. So, we still need protection from re-oxidation during soldering, as well as oxide removal. Protection from re-oxidation is done easily enough using a nitrogen atmosphere. Oxide reduction and removal can be accomplished by adding hydrogen to the atmosphere, otherwise known as forming gas. So, although we haven’t used a liquid chemical, the N2/H2 mix is a “chemical” and behaves as a flux.
The proportion of hydrogen is typically between 5-10% in conveyor/belt ovens, and can be as high as 100% in specialized equipment. Heat transfer is improved by higher H2 levels as the thermal capacity of hydrogen is about 14 times that of nitrogen. At higher H2 percentages the oxide reduction is faster but then safety precautions (flame traps etc.) may have to be used. Interestingly enough, at about 75% concentration, the hydrogen is no longer explosive as there is not enough oxygen to support combustion (although there are concerns for hydrogen embrittlement). A mix that has less than 5% hydrogen is non-flammable as the hydrogen is too dilute to burn. However, at that dilution the rate of hydrogen, oxide removal can be too slow to be practical.
It is also important to note that the rate of oxide reduction is enhanced by higher temperatures. In a production environment with Au/Sn solder, process temperatures are typically 330-350°C for good oxide reduction. With a fluxless soldering process, there is an absolute need for cleanliness as parts can be affected not just by oxide but surface contamination as well. Surface contamination can be introduced by handling during processing of the AuSn preforms as well as in use. Preforms are made in a mechanical process so great care needs to be exercised to minimize contamination. In addition, your solder supplier has to carefully control the thickness tolerance. AuSn “wets in place”; it does not spread when wetting. Any variation in thickness will tend to be present in finished joints. Choose your supplier carefully!
Next time we will look at reflow with fluxes including formic acid (which is not the same as forming gas).